Coupling of oxytocin and cholecystokinin pathways in the hypothalamus is required for gut-to-brain homeostatic feeding control
Abstract
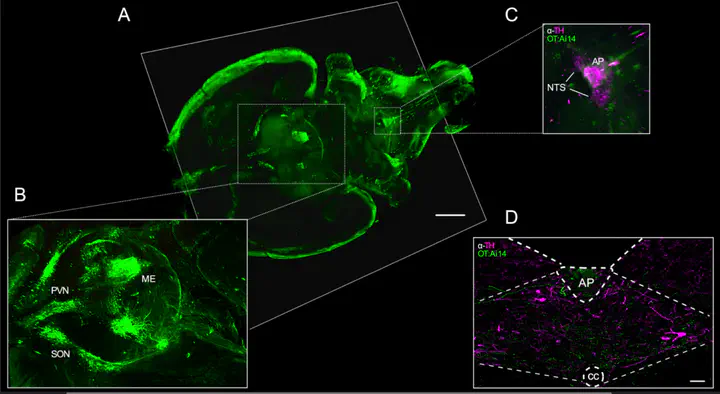
Oxytocin-expressing paraventricular hypothalamic neurons (PVNOT neurons) integrate afferent signals from the gut including cholecystokinin (CCK) to adjust whole-body energy homeostasis. However, the molecular underpinnings by which PVNOT neurons orchestrate gut-to-brain feeding control remain unclear. Here, we show that mice undergoing selective ablation of PVNOT neurons fail to reduce food intake in response to CCK and develop hyperphagic obesity on chow diet. Notably, exposing wildtype mice to a high-fat/high-sugar (HFHS) diet recapitulates this insensitivity towards CCK, which is linked to diet-induced transcriptional and electrophysiological aberrations specifically in PVNOT neurons. Restoring OT pathways in DIO mice via chemogenetics or polypharmacology sufficiently re-establishes CCK’s anorexigenic effects. Lastly, by single-cell profiling, we identify a specialized PVNOT neuronal subpopulation with increased κ-opioid signaling under HFHS diet, which restrains their CCK-evoked activation. In sum, we here document a novel (patho)mechanism by which PVNOT signaling uncouples a gut-brain satiation pathway under obesogenic conditions.